**Chemical Properties and Reactions**:
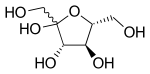
– Fructose is a 6-carbon polyhydroxyketone that adopts a cyclic structure in solution and exists as an equilibrium mixture of various tautomers.
– Fructose can be anaerobically fermented by yeast and bacteria, undergo Maillard reactions, and yield hydroxymethylfurfural upon dehydration.
– Fructose’s ability to rotate light makes it laevorotary, and it is known for its high sweetness and synergy with other sweeteners.
– Fructose has a greater impact on freezing point depression and enhances starch viscosity more rapidly than sucrose.
**Nutritional Information and Food Sources**:
– Fructose provides 368 kcal per 100 grams of dry powder and has 95% the caloric value of sucrose by weight.
– Natural sources of fructose include fruits, vegetables, and honey, and it is often concentrated from these sources.
– Fructose exists in foods as a free monosaccharide or bound to glucose as sucrose, with varying levels in different foods.
**Absorption, Metabolism, and Health Implications**:
– Fructose absorption occurs in the small intestine and is facilitated by specific carriers, with exercise impacting absorption and metabolism affecting glycogen concentrations.
– Excessive fructose consumption is linked to metabolic diseases, and fructose may impact endothelial function and overall health.
– Fructose metabolism involves phosphorylation by fructokinase and can lead to glycogen and triglyceride synthesis.
**Cardiometabolic Diseases and Regulation**:
– Excess fructose consumption is associated with an increased risk of obesity, diabetes, and cardiovascular disorders.
– Fructose is part of the metabolic syndrome, may increase triglycerides in type-2 diabetes, and has a lower glycemic index compared to glucose and sucrose.
– Regulations exist for labeling fructose content in food products, and understanding fructose content is essential for dietary planning.
**Research, Studies, and Additional Information**:
– Studies investigate the effects of fructose on glycemic response, carbohydrate metabolism, and its role in metabolic diseases.
– Research explores the sweetness levels of fructose compared to other sweeteners and continues to contribute to nutritional science.
– Additional information includes NMR studies on fructose tautomers, fructose’s synthesis into transportation fuels, and its detection in open-chain forms in solution.
This article's lead section contains information that is not included elsewhere in the article. (March 2023) |
Fructose (/ˈfrʌktoʊs, -oʊz/), or fruit sugar, is a ketonic simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion. The liver then converts both fructose and galactose into glucose, so that dissolved glucose, known as blood sugar, is the only monosaccharide present in circulating blood.
| |||
 Haworth projection of β-d-fructofuranose
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
D-arabino-Hex-2-ulose
| |||
| Systematic IUPAC name
(3S,4R,5R)-1,3,4,5,6-Pentahydroxyhexan-2-one | |||
| Other names
Fruit sugar, levulose, d-fructofuranose, d-fructose, d-arabino-hexulose
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.303 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H12O6 | |||
| Molar mass | 180.156 g·mol−1 | ||
| Density | 1.694 g/cm3 | ||
| Melting point | 103 °C (217 °F; 376 K) | ||
| ~4000 g/L (25 °C) | |||
| −102.60×10−6 cm3/mol | |||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
675.6 kcal/mol (2,827 kJ/mol) (Higher heating value) | ||
| Pharmacology | |||
| V06DC02 (WHO) | |||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
15000 mg/kg (intravenous, rabbit) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847. The name "fructose" was coined in 1857 by the English chemist William Allen Miller. Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars. Fructose is found in honey, tree and vine fruits, flowers, berries, and most root vegetables.
Commercially, fructose is derived from sugar cane, sugar beets, and maize. High-fructose corn syrup is a mixture of glucose and fructose as monosaccharides. Sucrose is a compound with one molecule of glucose covalently linked to one molecule of fructose. All forms of fructose, including those found in fruits and juices, are commonly added to foods and drinks for palatability and taste enhancement, and for browning of some foods, such as baked goods. As of 2004, about 240,000 tonnes of crystalline fructose were being produced annually.
Excessive consumption of sugars, including fructose, (especially from sugar-sweetened beverages) may contribute to insulin resistance, obesity, elevated LDL cholesterol and triglycerides, leading to metabolic syndrome. The European Food Safety Authority (EFSA) stated in 2011 that fructose may be preferable over sucrose and glucose in sugar-sweetened foods and beverages because of its lower effect on postprandial blood sugar levels, while also noting the potential downside that "high intakes of fructose may lead to metabolic complications such as dyslipidaemia, insulin resistance, and increased visceral adiposity". The UK's Scientific Advisory Committee on Nutrition in 2015 disputed the claims of fructose causing metabolic disorders, stating that "there is insufficient evidence to demonstrate that fructose intake, at levels consumed in the normal UK diet, leads to adverse health outcomes independent of any effects related to its presence as a component of total and free sugars."