**Chemical and Physical Properties**:
– Glucose forms white or colorless solids.
– Highly soluble in water and acetic acid.
– Poorly soluble in methanol and ethanol.
– Melts at 146°C and 150°C.
– Has a pKa value of 12.16 in water at 25°C.
**Synthesis, Production, and Structure**:
– Glucose can be obtained by hydrolysis of carbohydrates.
– Commercially manufactured from starches like corn, potato, wheat, and tapioca.
– Glucose is a monosaccharide with six carbon atoms and an aldehyde group.
– Glucose exists in solid form as a monohydrate with a closed pyran ring.
– Glucose is a building block of disaccharides like lactose and sucrose, and polysaccharides like starch and cellulose.
**Medical and Nutritional Significance**:
– Glucose is the most important source of energy in all organisms.
– Stored as starch in plants and glycogen in animals for metabolism.
– Glucose circulates in the blood as blood sugar.
– Intravenous sugar solution is on the WHO’s List of Essential Medicines.
– Glucose is used in combination with sodium chloride.
**Mutarotation and Optical Activity**:
– Glucose molecules undergo mutarotation, switching between open-chain and cyclic forms.
– Glucose exhibits optical activity and can rotate plane-polarized light.
– The equilibrium mixture of glucose stabilizes at an α:β ratio of 36:64.
– Glucose’s optical activity is a key characteristic in various chemical and biological processes.
– Glucose anomers can be observed in a polarimeter with specific rotation angles.
**Metabolism and Energy Source**:
– Glucose is produced by plants through photosynthesis and stored as polymers.
– Glucose is metabolized by glycolysis and the pentose phosphate pathway in humans.
– Glucose is a ubiquitous fuel used in aerobic respiration, anaerobic respiration, or fermentation.
– Insulin and other mechanisms regulate blood glucose concentration.
– Glucose and oxygen supply energy for the brain, influencing psychological processes.
Glucose is a sugar with the molecular formula C6H12O6. Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world.
 Skeletal formula of d-glucose
| |
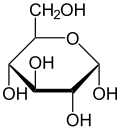 Haworth projection of α-d-glucopyranose
| |
 Fischer projection of d-glucose
| |
| Names | |
|---|---|
| Pronunciation | /ˈɡluːkoʊz/, /ɡluːkoʊs/ |
| IUPAC name
Allowed trivial names:
| |
| Preferred IUPAC name
PINs are not identified for natural products. | |
Systematic IUPAC name
| |
| Other names
Blood sugars
Dextrose Corn sugar d-Glucose Grape sugar | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| Abbreviations | Glc |
| 1281604 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 83256 | |
| KEGG | |
| MeSH | Glucose |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.156 g/mol |
| Appearance | White powder |
| Density | 1.54 g/cm3 |
| Melting point | α-d-Glucose: 146 °C (295 °F; 419 K) β-d-Glucose: 150 °C (302 °F; 423 K) |
| 909 g/L (25 °C (77 °F)) | |
| −101.5×10−6 cm3/mol | |
| 8.6827 | |
| Thermochemistry | |
Heat capacity (C)
|
218.6 J/(K·mol) |
Std molar
entropy (S⦵298) |
209.2 J/(K·mol) |
Std enthalpy of
formation (ΔfH⦵298) |
−1271 kJ/mol |
| 2,805 kJ/mol (670 kcal/mol) | |
| Pharmacology | |
| B05CX01 (WHO) V04CA02 (WHO), V06DC01 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | ICSC 08655 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is d-glucose, while its stereoisomer l-glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Glucose is naturally occurring and is found in its free state in fruits and other parts of plants. In animals, glucose is released from the breakdown of glycogen in a process known as glycogenolysis.
Glucose, as intravenous sugar solution, is on the World Health Organization's List of Essential Medicines. It is also on the list in combination with sodium chloride (table salt).
The name glucose is derived from Ancient Greek γλεῦκος (gleûkos, "wine, must"), from γλυκύς (glykýs, "sweet"). The suffix "-ose" is a chemical classifier denoting a sugar.

