**History of Ascorbic Acid:**
– James Lind demonstrated antiscorbutic properties of certain foods in the 18th century.
– Albert Szent-Györgyi isolated hexuronic acid in 1928-1932.
– Walter Norman Haworth deduced the correct structure of ascorbic acid in 1933.
– Haworth and Szent-Györgyi proposed renaming it ascorbic acid.
– Nobel Prizes in Chemistry and Physiology or Medicine awarded in 1937 for their work.
**Chemical Properties of Ascorbic Acid:**
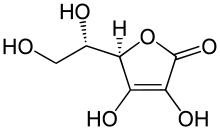
– Ascorbic acid is a furan-based lactone of 2-ketogluconic acid.
– Forms salts like sodium ascorbate.
– Can react with organic acids to form esters like ascorbyl palmitate.
– Oxidizes to form dehydroascorbic acid, a mild reducing agent and antioxidant.
**Uses of Ascorbic Acid:**
– Mainly used as a food additive to combat oxidation.
– Commonly used as a dietary supplement.
– Used in various industries such as photography, preservatives, and fluorescence microscopy.
– Intravenous high-dose ascorbate used in chemotherapy and as a urinary acidifier.
**Synthesis of Ascorbic Acid:**
– Natural biosynthesis occurs in many plants and animals.
– Industrial synthesis from glucose via the Reichstein process.
– Reichstein process involves steps like catalytic hydrogenation of glucose to sorbitol and oxidation to sorbose.
**Production and Determination of Ascorbic Acid:**
– Ascorbic acid production is prominent in China using biotechnological processes.
– Determination methods include traditional titration, iodometry, and alternative oxidizing agents.
– Various references and resources are available for further information on ascorbic acid.
Ascorbic acid is an organic compound with formula C
6H
8O
6, originally called hexuronic acid. It is a white solid, but impure samples can appear yellowish. It dissolves freely in water to give mildly acidic solutions. It is a mild reducing agent.
| |

| |
| Names | |
|---|---|
| IUPAC name
(5R)-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one
| |
| Other names
Vitamin C
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| E number | E300 (antioxidants, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H8O6 | |
| Molar mass | 176.124 g·mol−1 |
| Appearance | White or light yellow solid |
| Density | 1.65 g/cm3 |
| Melting point | 190 to 192 °C (374 to 378 °F; 463 to 465 K) decomposes |
| 330 g/L | |
| Solubility | Insoluble in diethyl ether, chloroform, benzene, petroleum ether, oils, fats |
| Solubility in ethanol | 20 g/L |
| Solubility in glycerol | 10 g/L |
| Solubility in propylene glycol | 50 g/L |
| Acidity (pKa) | 4.10 (first), 11.6 (second) |
| Pharmacology | |
| A11GA01 (WHO) G01AD03 (WHO), S01XA15 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
11.9 g/kg (oral, rat) |
| Safety data sheet (SDS) | JT Baker |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ascorbic acid exists as two enantiomers (mirror-image isomers), commonly denoted "l" (for "levo") and "d" (for "dextro"). The l isomer is the one most often encountered: it occurs naturally in many foods, and is one form ("vitamer") of vitamin C, an essential nutrient for humans and many animals. Deficiency of vitamin C causes scurvy, formerly a major disease of sailors in long sea voyages. It is used as a food additive and a dietary supplement for its antioxidant properties. The "d" form can be made via chemical synthesis, but has no significant biological role.

