**1. Chemical Properties and Structure of Lactose:**
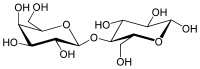
– Lactose is a disaccharide composed of glucose and galactose.
– Its systematic name is β-galactopyranosyl-(1→4)-glucose.
– Lactose is less sweet than sucrose and is soluble in water.
– It undergoes hydrolysis to break down into its monosaccharide components.
– The enzyme lactase is required to break down lactose in the digestive system.
**2. Occurrence, Isolation, and Industrial Uses of Lactose:**
– Lactose makes up about 2–8% of milk by weight.
– Several million tons of lactose are produced annually as a by-product of the dairy industry.
– Whey, containing 4.8% lactose, is purified by crystallisation.
– Lactose is used in the pharmaceutical industry as a filler in tablets.
– It is also utilized in the food industry for its sweetening properties and in the production of certain cheeses.
**3. Metabolism and Health Effects of Lactose:**
– Infant mammals produce lactase to digest lactose in milk.
– Lactase production decreases with maturity due to weaning.
– Over 70% of western Europeans can digest lactose as adults.
– Lactose intolerance can lead to gastrointestinal symptoms like bloating and diarrhea.
– Lactose-free products are available for those with lactose intolerance.
**4. Biological Properties and Applications of Lactose:**
– Lactose has a sweetness index of 0.2 to 0.4 and a caloric value of 4 kcal/g when fully digested.
– Undigested lactose acts as dietary fiber and aids in mineral absorption.
– Lactose is used as a carrier and stabilizer for aromas and pharmaceutical products.
– It is essential in infant formula to match human milk composition.
– Lactose is used in tablet and capsule drug products for its properties.
**5. Sources, Historical Significance, and Detection Reactions of Lactose:**
– Sources of lactose include dairy products, processed foods, medications, and supplements.
– Lactose was first isolated from milk in the 18th century.
– Scientists like Carl Wilhelm Scheele and Emil Fischer made significant contributions to understanding lactose.
– The configuration of glucose and galactose was determined by Fischer.
– Detection reactions for lactose include the Woehlk and Fearons test.
Lactose, or milk sugar, is a disaccharide sugar composed of galactose and glucose subunits and has the molecular formula C12H22O11. Lactose makes up around 2–8% of milk (by mass). The name comes from lact (gen. lactis), the Latin word for milk, plus the suffix -ose used to name sugars. The compound is a white, water-soluble, non-hygroscopic solid with a mildly sweet taste. It is used in the food industry.
| |
| Names | |
|---|---|
| IUPAC name
β-D-Galactopyranosyl-(1→4)-D-glucose
| |
| Systematic IUPAC name
(2R,3R,4S,5R,6S)-2-(Hydroxymethyl)-6-{[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}oxane-3,4,5-triol | |
| Other names
Milk sugar
Lactobiose 4-O-β-D-Galactopyranosyl-D-glucose | |
| Identifiers | |
3D model (JSmol)
|
|
| 90841 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.509 |
| EC Number |
|
| 342369 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.297 g·mol−1 |
| Appearance | White solid |
| Density | 1.525 g/cm3 |
| Melting point | 252 °C (anhydrous) 202 °C (monohydrate) |
| 195 g/L | |
Chiral rotation ([α]D)
|
+55.4° (anhydrous) +52.3° (monohydrate) |
| Thermochemistry | |
Std enthalpy of
combustion (ΔcH⦵298) |
5652 kJ/mol, 1351 kcal/mol, 16.5 kJ/g, 3.94 kcal/g |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 357.8 °C (676.0 °F; 631.0 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

