History:
– Discovered by Augustin-Pierre Dubrunfaut
– Confirmation by Cornelius OSullivan in 1872
– Name derived from malt and suffix -ose
– Key role in starch structure
– Named after malt due to its presence in germinating seeds
Structure and nomenclature:
– Classified as a disaccharide
– Two glucose units in pyranose form
– Joined by O-glycosidic bond (1→4)
– Differentiated as α-maltose or β-maltose
– Isomer is isomaltose with α(1→6) bond
Properties:
– Reducing sugar like glucose
– Broken down to glucose by maltase enzyme
– Exhibits mutarotation in aqueous solution
– Detectable by Woehlk or Fearons test
– Less sweet than sucrose, about 30-60%
Sources and absorption:
– Present in maltose syrup and partially hydrolyzed starch products
– Found in malt component from softened grain
– Broken down by maltase enzymes in humans
– Provides glucose molecules for energy or glycogen storage
– Rare maltose intolerance due to multiple maltase enzymes
References:
– CRC Handbook of Chemistry and Physics
– Organic and Biological Chemistry
– Journal of the Chemical Society
– Food Chemistry
– CRC Handbook of Food Additives
Maltose (/ˈmɔːltoʊs/ or /ˈmɔːltoʊz/), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar.
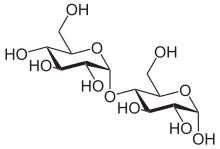 α-Maltose
| |
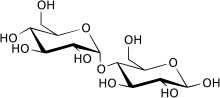 β-Maltose
| |
| Names | |
|---|---|
| IUPAC name
4-O-α-D-Glucopyranosyl-D-glucose
| |
| Systematic IUPAC name
(3R,4R,5S,6R)-6-(hydroxymethyl)-5-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxane-2,3,4-triol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.651 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.297 g·mol−1 |
| Appearance | White powder or crystals |
| Density | 1.54 g/cm3 |
| Melting point | 160 to 165 °C (320 to 329 °F; 433 to 438 K) (anhydrous) 102–103 °C (monohydrate) |
| 1.080 g/mL (20 °C) | |
Chiral rotation ([α]D)
|
+140.7° (H2O, c = 10) |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related
|
Sucrose Lactose Trehalose Cellobiose |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

