**Chemical Properties and Uses**:
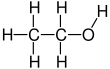
– Ethanol has the chemical formula CH3OH.
– Justus Liebig coined the name ‘ethyl’ in 1834, and ‘ethanol’ was termed in 1892.
– It is used as an anesthetic, antiseptic, solvent for medications, and in over 700 liquid medicine preparations.
– Ethanol acts as an antidote for certain poisonings.
– It was historically used as a general anesthetic and is effective against bacteria, fungi, and viruses.
**Recreational and Medical Applications**:
– Ethanol is a commonly consumed psychoactive drug and a central nervous system depressant.
– It is legal for sale in most countries but is addictive and carcinogenic.
– Laws regulate the sale and consumption of alcoholic beverages.
– Ethanol can be administered as an antidote for specific poisonings.
– It is metabolized in the liver and stomach by ADH enzymes.
**Industrial and Fuel Applications**:
– Ethanol is widely used as engine fuel and a fuel additive.
– Brazil heavily relies on ethanol as engine fuel, and the US primarily uses E10 and E85 ethanol/gasoline mixtures.
– Ethanol reduces harmful tailpipe emissions and has a higher research octane number than gasoline.
– It was historically used in rocket fuels and is currently used in lightweight rocket-powered aircraft.
– Ethanol is attractive for fuel cells due to availability, cost, purity, and low toxicity.
**Household and Chemical Applications**:
– Ethanol is used in household heating, cooking, and as a versatile solvent in paints, tinctures, and personal care products.
– It is used in DNA and RNA purification through ethanol precipitation.
– Ethanol is a crucial industrial ingredient and a precursor for various organic compounds.
– It plays a significant role in chemical reactions and the production of ethyl halides, esters, and acetic acid.
– Ethanol is also used in the synthesis of ethyl amines.
**Physical and Natural Properties**:
– Ethanol is a volatile, colorless liquid with a slight odor that burns with a smokeless blue flame.
– It has a refractive index slightly higher than water and a triple point at 150 ± 20 K.
– Ethanol is miscible with water and many organic solvents, forming azeotropes at specific concentrations.
– It catches fire above its flash point and is considered flammable in concentrations above 2.35% by mass.
– Ethanol is a byproduct of yeast metabolism, found in overripe fruit, and produced during the germination of many plants.
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula CH3CH2OH. It is an alcohol, with its formula also written as C2H5OH, C2H6O or EtOH, where Et stands for ethyl. Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, and the active ingredient in alcoholic drinks.
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Pronunciation | /ˈɛθənɒl/ | ||
| Preferred IUPAC name
Ethanol | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 1718733 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.526 | ||
| 787 | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| UN number | UN 1170 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H6O | |||
| Molar mass | 46.069 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | wine-like, pungent | ||
| Density | 0.78945 g/cm3 (at 20 °C) | ||
| Melting point | −114.14 ± 0.03 °C (−173.45 ± 0.05 °F; 159.01 ± 0.03 K) | ||
| Boiling point | 78.23 ± 0.09 °C (172.81 ± 0.16 °F; 351.38 ± 0.09 K) | ||
| Miscible | |||
| log P | −0.18 | ||
| Vapor pressure | 5.95 kPa (at 20 °C) | ||
| Acidity (pKa) | 15.9 (H2O), 29.8 (DMSO) | ||
| −33.60·10−6 cm3/mol | |||
Refractive index (nD)
|
1.3611 | ||
| Viscosity | 1.2 mPa·s (at 20 °C), 1.074 mPa·s (at 25 °C) | ||
| 1.69 D | |||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H225, H319, H360D | |||
| P210, P233, P240, P241, P242, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 14 °C (Absolute) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1000 ppm (1900 mg/m3) | ||
REL (Recommended)
|
TWA 1000 ppm (1900 mg/m3) | ||
IDLH (Immediate danger)
|
3300 ppm | ||
| Safety data sheet (SDS) | |||
| Related compounds | |||
Related compounds
|
|||
| Supplementary data page | |||
| Ethanol (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was 51 gigalitres (1.3×1010 US gallons), coming mostly from Brazil and the U.S.