**Medical Uses of Quinine:**
– Used to treat malaria, babesiosis, lupus, and arthritis.
– Recommended in chloroquine-resistant malaria cases.
– Available in forms like sulfate and gluconate.
– No longer WHO’s first-line malaria treatment.
– Acts as an MAO inhibitor.
– Found in tonic water for its bitter taste.
**Mechanism of Action and Chemistry of Quinine:**
– Toxic to Plasmodium falciparum by interfering with hemoglobin metabolism.
– Inhibits glycolysis and hemozoin biocrystallization in parasites.
– Targets malaria purine nucleoside phosphorylase enzyme.
– UV absorption peaks at 350nm, fluorescent emission peaks at 460nm.
– Highly fluorescent in sulfuric acid solution.
**Adverse Effects and Contraindications of Quinine:**
– Can induce severe adverse effects in multiple organ systems.
– May cause heart arrhythmias and hemolysis in G6PD deficiency.
– Increases plasma concentrations of CYP2D6 metabolized drugs.
– Serious reactions include low platelet count and kidney failure.
– Adverse effects like torsades de pointes, blackwater fever, and neutropenia.
**History and Biosynthesis of Quinine:**
– Originally sourced from cinchona trees.
– Formal chemical synthesis achieved in 1944.
– Biosynthesis involves strictosidine synthase and modification steps.
– Played a significant role in European colonization and malaria treatment.
– Used by Quechua people, introduced to Europe by Jesuit missionaries.
**Regulation, Usage, and Miscellaneous Information:**
– FDA warnings on health risks related to quinine.
– Historical significance in treating malaria.
– Used in tonic water and as a food additive.
– Synthetic production less economically viable than natural isolation.
– Listed as a cutting agent in street drugs, impacting drug potency and posing health risks.
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to Plasmodium falciparum that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg cramps, quinine is not recommended for this purpose due to the risk of serious side effects. It can be taken by mouth or intravenously. Malaria resistance to quinine occurs in certain areas of the world. Quinine is also used as an ingredient in tonic water to impart a bitter taste.
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | US: /ˈkwaɪnaɪn/, /kwɪˈniːn/ or UK: /ˈkwɪniːn/ KWIN-een |
| Trade names | Qualaquin, Quinbisul, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682322 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular, intravenous, rectal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 70–95% |
| Metabolism | Liver (mostly CYP3A4 and CYP2C19-mediated) |
| Elimination half-life | 8–14 hours (adults), 6–12 hours (children) |
| Excretion | Kidney (20%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.550 |
| Chemical and physical data | |
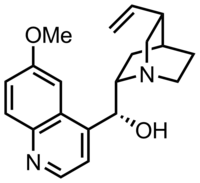
| Formula | C20H24N2O2 |
| Molar mass | 324.424 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 177 °C (351 °F) |
| |
| |
| | |
Common side effects include headache, ringing in the ears, vision issues, and sweating. More severe side effects include deafness, low blood platelets, and an irregular heartbeat. Use can make one more prone to sunburn. While it is unclear if use during pregnancy carries potential for fetal harm, treating malaria during pregnancy with quinine when appropriate is still recommended. Quinine is an alkaloid, a naturally occurring chemical compound. How it works as a medicine is not entirely clear.
Quinine was first isolated in 1820 from the bark of a cinchona tree, which is native to Peru, and its molecular formula was determined by Adolph Strecker in 1854. The class of chemical compounds to which it belongs is thus called the cinchona alkaloids. Bark extracts had been used to treat malaria since at least 1632 and it was introduced to Spain as early as 1636 by Jesuit missionaries returning from the New World. It is on the World Health Organization's List of Essential Medicines. Treatment of malaria with quinine marks the first known use of a chemical compound to treat an infectious disease.
