**Historical Evolution of Distillation**
– Early evidence of distillation found on Akkadian tablets
– Aristotle’s experiment with seawater evaporation
– Distillation practiced in ancient India, China, and by Alexandrian chemists
– Distillation in the Islamic Golden Age
– Evolution from Alchemy to Chemistry
– Alchemy transitioned into chemistry
– Retorts and alembics were used for distillations
– Copper alembics were later invented
– Cooling systems in alembics improved alcohol condensation efficiency
**Types of Distillation Equipment**
– Alembics and retorts were early distillation vessels
– Alembics had air-cooled condensers
– Copper alembics and pot stills were developed
– Pot stills had cooling systems for efficient condensation
– Modern industrial processes have more efficient distillation methods
**Applications and Significance of Distillation**
– Distilled water usage since c.200 CE
– Distillation of other liquids in early Byzantine Egypt
– Distillation experiments by medieval Muslim chemists
– Distillation of wine in Arabic works
– Fractional distillation in organic substances
– Applications of Pot Stills
– Pot stills are used for producing fine alcohols
– Common alcohols made in pot stills include cognac, whiskey, tequila
– Materials used for pot stills include wood, clay, stainless steel
– Pot stills are still widely used despite technological advancements
– Some vodkas and rums are also made using pot stills
– Historical Significance of Distillation
– Distillation has a long history dating back centuries
– It played a crucial role in the evolution of alchemy to chemistry
– Distillation vessels like retorts and alembics were key tools
– The process of distillation has been refined over time
– Distillation techniques have been passed down through generations
**Distillation in Ancient China**
– Distillation of beverages started in the Southern Song and Jin dynasties
– Archaeological evidence of distillation in Qinglong, Hebei province
– Distilled beverages common during the Yuan dynasty
– Alcohol distillation during the 10th–14th centuries
– Hieronymus Brunschwig’s book on distillation in 1500
**Modern Era and Trends in Distillation**
– German alchemist published the first book solely on distillation
– John French’s major English compendium on distillation
– Industrial scale diagrams in distillation publications
– Evolution of distillation practices over time
– Contributions of key figures in the modern era
– Modern Trends in Distillation
– Industrial processes now use more efficient distillation methods
– Traditional pot stills are still preferred for certain alcohols
– Materials like stainless steel are now commonly used in distillation
– Distillation technology continues to evolve
– Distillation remains a fundamental process in alcohol production
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.
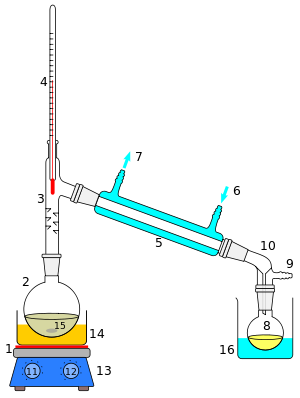
1: The heat source to boil the mixture
2: round-bottom flask containing the mixture to be boiled
3: the head of the still
4: mixture boling-point thermometre
5: the condenser of the still
6: the cooling-water inlet of the condenser
7: the cooling-water outlet of the condenser
8: the distillate-receiving flask
9: vacuum pump and gas inlet
10: the receiver of the still
11: the heat control for heating the mixture
12: stirring mechanism speed control
13: stirring mechanism and heating plate
14: heating bath (oil/sand) for the flask
15: the stirring mechanism (not shown, e.g. boiling chips or mechanical stirring machine)
16: the distillate-cooling water bath.
Dry distillation (thermolysis and pyrolysis) is the heating of solid materials to produce gases that condense either into fluid products or into solid products. The term dry distillation includes the separation processes of destructive distillation and of chemical cracking, breaking down large hydrocarbon molecules into smaller hydrocarbon molecules. Moreover, a partial distillation results in partial separations of the mixture's components, which process yields nearly-pure components; partial distillation also realizes partial separations of the mixture to increase the concentrations of selected components. In either method, the separation process of distillation exploits the differences in the relative volatility of the component substances of the heated mixture.
In the industrial applications of classical distillation, the term distillation is used as a unit of operation that identifies and denotes a process of physical separation, not a chemical reaction; thus an industrial installation that produces distilled beverages, is a distillery of alcohol. These are some applications of the chemical separation process that is distillation:
- Distilling fermented products to yield alcoholic beverages with a high content by volume of ethyl alcohol.
- Desalination to produce potable water and for medico-industrial applications.
- Crude oil stabilisation, a partial distillation to reduce the vapor pressure of crude oil, which thus is safe to store and to transport, and thereby reduces the volume of atmospheric emissions of volatile hydrocarbons.
- Fractional distillation used in the midstream operations of an oil refinery for producing fuels and chemical raw materials for livestock feed.
- Cryogenic Air separation into the component gases — oxygen, nitrogen, and argon — for use as industrial gases.
- Chemical synthesis to separate impurities and unreacted materials.
